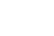Introduction
TruMonitor aligns with WHO PQS Performance Specification E006/TR03.2 and US FDA 21CFR Title 11 as IoT based remote Vaccine Cold Chain monitoring system. Covishied and Covaxin need to be maintained between 2-80C while Sputnik-V needs to be maintained below -180 C before administration. The importance of real time monitoring to prevent vaccine spoilage has been highlighted in various studies by multiple government as well as research organisations.
If Covid-19 vaccines are subjected to irregular temperatures, there is no way of knowing if the administered vaccines are active or effective. Using a real time monitoring system is essential for technicians to take corrective steps and prevent temperature swings.
Use of TruMonitor, an intelligent real-time temperature monitoring system will create a seamless monitoring regimen while keeping everyone involved in the loop. If implemented at state or divisional level, all concerned officers, from local municipal and district health officers to FDA inspectors can be given access to the system and the App, reducing their burden of inspection and monitoring.
Current Problems and Solutions
| Sr. No. | Current Problems | TruMonitor Solution |
|---|---|---|
| 1 | Covishield and Covaxin require 2 to 80C storage while Sputnik-V requires -18 0C storage until administration | TruMonitor allows settable Alarm and Warning levels for each device. |
| 2 | Lack of appropriate devices. | Indigenously developed devices connected to the same cloud are cheaper than similar imported systems. |
| 3 | Data Loggers or manual readings show lack of temperature adherence after it has already ruined the vaccines | Warnings and alarms on device as well as App ensure that preventive action can be taken. |
| 4 | Manual measurements and records are not always authentic | Measurements and records are automated on the cloud. |
| 5 | Officers must rely on juniors for reports. | Officers are always kept in the loop via automatic Escalations and Automatic Reports |
| 6 | Impossible to know the situation in real time. | Real time temperature monitoring from anywhere in the world. |
| 7 | All records must be maintained manually by already short-staffed organisations. | Tedious record keeping is done automatically and stored on server for five years. |
| 8 | FDA inspectors must examine each record manually | MH-FDA inspectors can get concise reports of all the alarms and warnings combined and can focus only on problematic areas |
Several problems are identified by the Ministry of Health and Family Welfare, GoI. Some of the problems are reproduced below. None of these findings would be possible and corrective action taken without expensive imported automated data loggers and report generation.
Problem 1: Lack of Compliance and Record Keeping
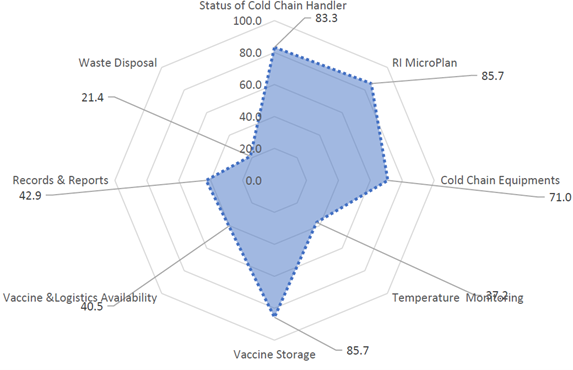
Fig. 1: State Vaccine Store, Bhojpur had only 37% Temperature Monitoring Compliance. Reproduced from “In depth analysis of cold chain, vaccine supply and logistics management for routine immunisation in three Indian states: an INCLEN program evaluation network study.”
Problem 2: Undetected Temperature Variations
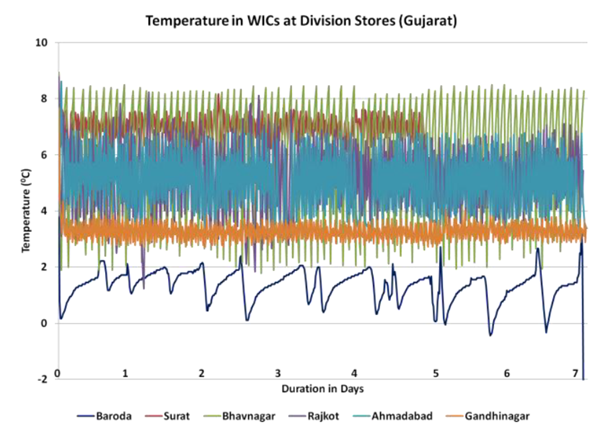
Fig. 2: Wide fluctuations and near constant <20C temperature at Baroda Division Store. reproduced from “In depth analysis of cold chain, vaccine supply and logistics management for routine immunisation in three Indian states: an INCLEN program evaluation network study.”
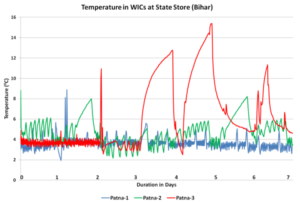
Fig. 3: Almost constant >80 C temperature in State Store 3 in Patana, reproduced from “In depth analysis of cold chain, vaccine supply and logistics management for routine immunisation in three Indian states: an INCLEN program evaluation network study.”
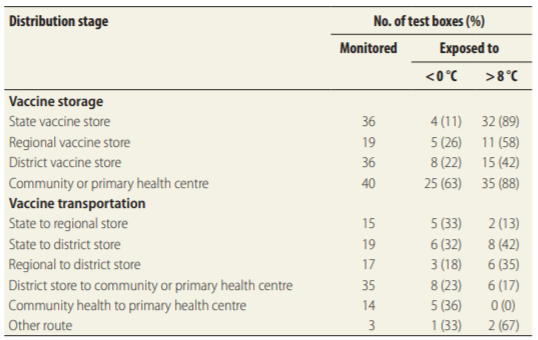
Problem 3: Undetected Temperature Variations at Community Healthcare Centers
Fig. 4: Suboptimal temperatures in the cold chain system for vaccines in India. Reproduced from “Frequent exposure to suboptimal temperatures in vaccine cold-chain system in India: results of temperature monitoring in 10 states.” By Murhekar MV, Srihari Dutta et. al.
Overview of TruMonitor
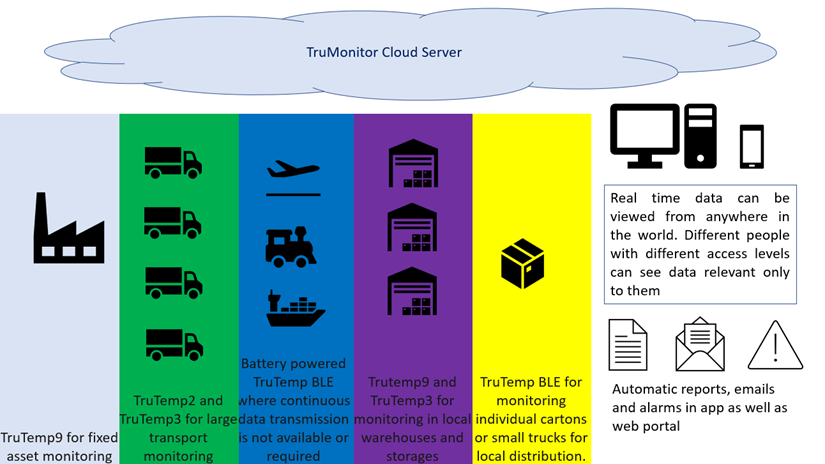
TruMonitor system consists of the following subcomponents which together form a complete system for cold chain monitoring.
TruMonitor system consists of electronics data loggers with cloud-based computer and mobile application which is an entirely automated monitoring system with minimum user intervention. Through this we aim to achieve the following:
- For stationary units and mobile units, TruMonitor data loggers will always keep monitoring the temperature. Additionally, temperature will be logged at intervals set by the user on the Cloud Server.
- If the temperature goes above or below the set temperature, warnings and alarms via emails and app will be used to communicate the discrepancy to the concerned users.
- Every Asset is mapped to a particular set of users who will oversee its operation and maintenance. To achieve this, TruMonitor has a hierarchy of users ranging from super admin to asset admin.
TruMonitor Hardware
To effectively cater to all segments of the cold chain, following data logger modules have been considered. Following table lists the basic features of the data loggers:
| Sr. No. | Data Logger | No. of probes | Connectivity and basic features |
|---|---|---|---|
| 1 | TruTemp Bluetooth | 1 digital probe | Connects via Bluetooth to the app. Battery powered. Continuous operations mode or data logging mode. Can work only with associated app. Cheapest module. |
| 2 | TruTemp 3 | 3 digital probes | WiFi plus GPRS connectivity, colour display changes colour with warning and alarm levels. 3G or 4G can be incorporated if required. |
| 3 | TruTemp 9x | 9 PT1000 probes, ambient Temp and Humidity | Ethernet and WiFi connectivity. Touch screen with USB ports for PDF reports. This module allows local report printing in addition to uploading data to the Cloud. Multiple 9 channel modules can be daisy chained together. |
Each device has an accuracy better than ±0.50 C and resolution of 0.10 C which confirms with or exceeds the requirements laid down by the WHO. The battery capacities are customizable and can be configured as per the user requirements.
TruMonitor Software
Software for TruMonitor consists of the TruMonitor App and the Web Portal as the front-end user interfaces supported by cloud database and analysis as Bask-End.
1. Hierarchical Structure
Each organisation can be divided into up to 5 levels viz. L4, L3, L2, L1, Asset and Sensor. System Administrators can set different levels of ‘Read+Write’ or ‘Read Only’ privileges to different users. For example, system administrators at the National level must have full privileges for the entire country, while system administrators at the State level must have full privileges but restricted for their State. Some officials must have read/write privileges, while others may only have read only privileges.
2. Dashboard
Each user, upon login, is taken to the dashboard of the area under his care. The dashboard shows the status of various assets under his care in a visual way with charts showing the overall performance of Warnings and Alarms etc. Customisation to the dashboards can also be provided for clients such as Map overlays of assets or any other additional information which might be required.
3. Automatic Reports
Users can get automatic reports of the logged data along with the warnings, alarms and escalations at the selected reporting frequency. The report PDFs are generated and emailed to the intended recipients automatically through the back end.
Additional custom reports to give an overview of the entire cold chain performance can be configured and they can also be sent automatically to intended recipients.
4. Warnings, Alarms and Escalations
Warnings, alarms and escalations can be set by the administrators with ‘Read+Write’ access to the assets under their care. These limits are set in the web portal or through the App and are automatically downloaded to the monitoring devices.
- For each sensor, the user can set warning and alarm limits for that parameter.
- During operation if any time the parameter crosses the set limit, app will show a notification of the warning.
- If the parameter crosses the alarm limit an Alarm LED will flash on the unit along with a buzzer and the parameter will be uploaded to the server.
- The server will send an email to the concerned email IDs. Simultaneously alarm will be displayed on the app and can be snoozed but cannot be deactivated until the parameter is brought into the limit.
- If the parameter is not brought within limit in the specified time frame, the alarm will automatically escalate to the supervisor.
5. Raw Data and APIs
Raw data for each sensor is retained for five years and can be downloaded in either CSV or PDF formats. This data can then be used for analysis using other third party softwares. APIs can be developed to share data at server level with existing client systems or to give data directly into government systems.
Applications of TruMonitor
By taking full advantage of proliferation of mobile networks and the internet, the temperature monitoring performance of the vaccine cold chain monitoring can be improved. The improvement takes place through automation, accountability, and the reliability of data. Following distinct applications of TruMonitor are considered:
- Vaccine Cold Chain– Government operated vaccination centres can be supplied with TruMonitor devices. The temperature data of the entire cold chain can be monitored and recorded centrally by concerned officers. Vaccine cold chain, especially at the local level is the weakest which can be helped by utilising the TruTemp3 devices connected to individual Insulated Boxes.
- Blood Banks– Blood Banks must ensure proper temperature controls and monitoring as per Maharashtra FDA guidelines so that the blood given to the patients is safe. A traditional central monitoring system still requires a lot of human intervention. At the same time, the temperature data at blood donation camps as well as during dispatch from the Bank to the Patient is rarely captured. With the implementation of the TruMonitor system, all of this data is collected together ensuring that each bag of blood is always maintained as per recommended standards.
- Hospital Pharmacies– NABH Accreditation requires temperature data logging of all temperature sensitive medicines which can be easily done through the 3 channel TruTemp3 devices. Even at non NABH accredited hospitals and clinics, recording the temperature data is the recommended practice to ensure patient safety.